-
5e
CHAPTERSodium-glucose Cotransporter-2 Inhibitors Beyond Glycemia
Amit Varma
ABSTRACT
Diabetes mellitus has emerged as the epidemic of the century, affecting millions and not respecting any geographical barriers. It should be viewed as a concept rather than a disease, affecting almost all organ systems, and may coexist with other unrelated conditions. Sodium-glucose cotransporter-2 (SGLT-2) inhibitors, as a class of drugs, have been found to act on multiple facets. They were primarily discovered as novel insulin-sparing anti-diabetic medication; however, they have shown promise beyond glycemic control. There are robust evidences emerging regarding their role in cardiovascular and renal outcomes by varied mechanisms.
INTRODUCTION
Phlorizin, a bitter white glycoside isolated from apple tree bark by French chemists in 1835, is a naturally occurring inhibitor of both sodium-glucose cotransporter-1 (SGLT-1) and SGLT-2 and was used for the treatment of diabetes in the preinsulin era. It was probably the first SGLT inhibitor known. The very idea of the discovery of this class came from a rare inherited condition, familial renal glycosuria, caused by a mutation in the SGLT-2 gene. Patients with this condition have varying degrees of glycosuria, remain asymptomatic, do not become dehydrated or hypoglycemic, and can excrete up to 125 g of glucose per day.
MECHANISM OF ACTION
Sodium-glucose cotransporter-2 inhibitors work by inhibiting the sodium-glucose cotransporter subtype-2, located in the S1 and S2 segments of the proximal convoluted tubule (PCT) of the kidney. SGLT-2 is a low-affinity, high-capacity transporter (2 mmol.mg.min) responsible for approximately 90% reabsorption of filtered plasma glucose, and the remainder is reabsorbed by SGLT-1. Potent SGLT-2 inhibition prevents the reabsorption of filtered glucose as well as sodium, resulting in glucosuria and natriuresis (Fig. 1 and Flowchart 1).
There are many SGLT receptors known, but 1 and 2 are the most important (Table 1).
CARDIOVASCULAR OUTCOMES
People with type 2 diabetes mellitus (T2DM) are 2.5 times at a higher risk of developing heart failure (HF) than nondiabetic patients. HF has been recognized as one of the earliest and common complications of various atherosclerotic cardiac and noncardiac events like myocardial infarction (MI), ischemic stroke, and limb ischemia.
Robust evidences were observed in a few landmark trials with relation to cardiovascular (CV) outcomes which are as follows:
- EMPA-REG OUTCOME trial
- CANVAS trial
- DECLARE-TIMI 58
- VERTIS-CV
EMPA-REG OUTCOME Trial
In 2015, EMPA-REG OUTCOME trial was the first Food and Drug Administration (FDA)-mandated cardiovascular outcome trial (CVOT) of the class. It studied 7,020 individuals with T2DM, randomized to empagliflozin or placebo over a mean follow-up period of 3.1 years. It comprehensively showed a reduction in major adverse cardiovascular events (MACE), number of events, death from CV causes or any other cause, and number of hospitalizations.
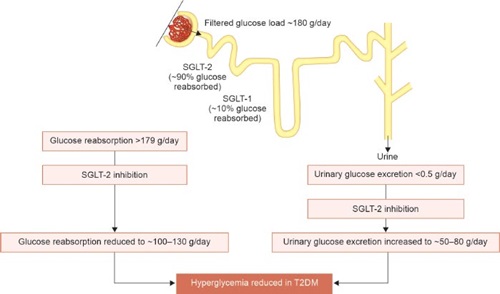
FIG. 1: Mechanism of action of SGLT-2 inhibitors.
(SGLT2: sodium-glucose cotransporter-2; T2DM: type 2 diabetes mellitus)
CANVAS
In the CANVAS (Canagliflozin Cardiovascular Assessment Study) program, patients with T2DM and high CV risk treated with canagliflozin had a significantly lower risk of the composite outcome of CV death, nonfatal MI, or nonfatal stroke; hospitalization for HF; and renal outcomes, but also a greater risk of lower-limb amputation.
Canagliflozin significantly reduced three-point MACE [hazard ratio (HR) 0.86; 95% confidence interval (CI) 0.75–0.97] and HF hospitalization (HR 0.67; 95% CI 0.52–0.87), but there was no significant reduction in mortality. This was largely due to the fact that majority had an established CV disease (Fig. 2).
DECLARE-TIMI 58
In a posthoc analysis of DECLARE-TIMI 58 where participants were stratified by ejection fraction (EF), dapagliflozin reduced the risk of CV death and HF hospitalization to a greater extent in patients with reduced EF.
VERTIS CV Trial
The VERTIS CV trial demonstrated the efficacy of ertugliflozin in reducing HF hospitalizations in patients with T2DM, similar to that seen with other SGLT-2 inhibitors. Empagliflozin and canagliflozin remain the only two SGLT-2 inhibitors that have shown a reduction in MACE in patients with T2DM in randomized clinical trials.
RENAL OUTCOME
By inhibiting sodium resorption in the proximal nephron, thereby increasing distal delivery, SGLT-2 inhibitors can restore tubuloglomerular feedback and normalize renal blood flow. The preferential effect of SGLT-2 inhibition on the afferent, rather than efferent, arteriole may explain why renal function returns to baseline after an initial dip in the estimated glomerular filtration rate (eGFR).
CREDENCE Trial
The CREDENCE (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation) trial was aimed to assess the effects of the SGLT-2 inhibitor, canagliflozin, on clinically important renal outcomes in people with T2DM and established chronic kidney disease (CKD). It was a multicenter, double-blinded, placebo-controlled randomized trial across 690 centers spanning 34 countries. It included subjects with T2DM with eGFR 30–90 mL/min/1.73 m2 and urine albumin-creatinine ratio (UACR) 300–5,000 mg/g, who are receiving standard of care including a maximum tolerated dose of angiotensin-converting enzyme (ACE) inhibitor and angiotensin II receptor blockers (ARBs), to assess whether canagliflozin compared with placebo reduces the composite outcome of end-stage kidney disease (ESKD), doubling of serum creatinine, or renal or CV death. The secondary objectives included CV death or hospitalization for HF, major CV events (3-point MACE: CV death, MI, or stroke), hospitalization for heart failure, ESKD, doubling of serum creatinine, or renal death, all-cause mortality or hospitalization for unstable angina.
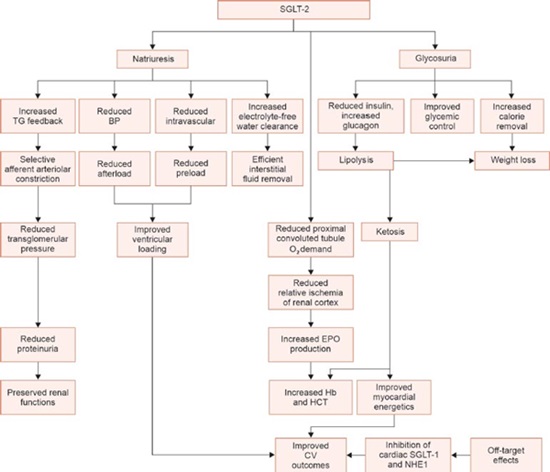
FLOWCHART 1: Effects of SGLT-2 inhibition.
(BP: blood pressure; CV: cardiovascular; EPO: erythropoietin; NHE1: Na+/H+ exchanger 1; PCT: proximal convoluted tubule; SGLT-1: sodium-glucose cotransporter-1; SGLT-2: sodium-glucose cotransporter-2; TG: tubuloglomerular)
TABLE 1: A comparison of selected characteristics of SGLT-1 and SGLT-2. SGLT-1
SGLT-2
Site
Mostly small intestine, some in kidney and heart
Almost exclusively kidney
Renal location
Late PCT (S3 segment)
Early PCT (S1 segment)
Affinity for glucose
High (Km = 0.4 mm)
Low (Km = 2 mm)
Capacity for glucose transport
Low
High
Percentage of real glucose reabsorption
10
90
(PCT: proximal convoluted tubule; SGLT-1: sodium-glucose cotransporter-1; SGLT-2: sodium-glucose cotransporter-2) 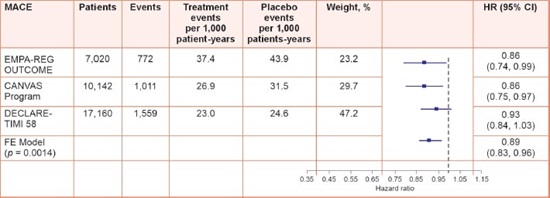
FIG. 2: Pooled data for the composite of MACE, MI, stroke, and CV death.
(CI: confidence interval; CV: cardiovascular; HR: hazard ratio; MACE: major adverse cardiovascular events; MI: myocardial infarction)
Canagliflozin reduced the risk of the primary outcome of ESKD, doubling of serum creatinine, or renal or CV death by 30% (p = 0.00001). The results were consistent across a broad range of prespecified subgroups. Canagliflozin also reduced the risk of the secondary outcome of ESKD, doubling of serum creatinine, or renal death by 34% (p < 0.001). Similar risk reductions were seen for exploratory outcomes assessing components of the primary outcome; ESKD was 32% lower and dialysis, transplantation, or renal death was 28% lower. Canagliflozin attenuated the slope of chronic eGFR decline by 2.7 mL/min/1.73 m2/year (1.9 vs. 4.6).
Cardiorenal Syndrome: An Unmet Need in Heart Failure
Up to 60% of patients with HF have comorbid CKD. By inhibiting sodium resorption in the proximal nephron, thereby increasing distal delivery, SGLT-2 inhibitors can restore tubuloglomerular feedback and normalize renal blood flow. The preferential effect of SGLT-2 inhibition on the afferent, rather than efferent, arteriole may explain why renal function returns to baseline after an initial dip in eGFR.
DAPA-CKD Trial
The DAPA-CKD (dapagliflozin and prevention of adverse outcomes in CKD) is the first dedicated clinical trial to explore the potential benefits and risks of SGLT-2 inhibitors in patients across multiple CKD stages, both with and without diabetes, who are already receiving evidence-based renoprotective therapy. It was a randomized, double-blinded, placebo-controlled, multicentric trial. It included subjects above 18 years of age, with eGFR between 25 and 75 mL/min/1.73 m2 and UACR between 200 and 500 mg/g who are on a maximal tolerated dose of ACE inhibitor or ARB.
The subjects had a lower risk of primary composite outcome in the sustained decline of eGFR of at least 50%, ESKD, or death from renal or CV causes than the participants in the placebo arm. There were no extra events of ketoacidosis or hypoglycemia as compared to placebo. There was also a lower risk of HF or death from CV causes. The initial dip in eGFR followed by stabilization of kidney function decline is seen with dapagliflozin, like other members of this class and ACE inhibitors.
MECHANISTIC ROLE OF SODIUM-GLUCOSE COTRANSPORTER-2 INHIBITORS
A characteristic feature of the pathophysiology of HF is the activation of compensatory pathways; however, sustained activation of the neurohormonal system (sympathetic nervous system and renin–angiotensin–aldosterone system) results in maladaptive remodeling of the ventricles and myocardial injury, which perpetuate the disease state.
The REFORM (REducing Falls with ORthoses and a Multifaceted podiatry intervention) trial evaluated left ventricular remodeling in patients, with HF and diabetes mellitus, on dapagliflozin. It showed a modest effect as the subjects in the study were already on the maximal dosage of diuretics, ACE inhibitors, and ARBs. Hence, they have maximal benefits in the early course of the disease.
Calcium and Cardiomyocyte
Calcium (Ca2+) is a key ion involved in excitation-contraction coupling and cardiac rhythmicity. It also acts as a second messenger in regulating gene transcription for myocyte hypertrophy and other pathological remodeling pathways. SGLT-2 is not expressed on the heart, yet there is strong preclinical evidence that SGLT-2 inhibitors influence Ca2+ handling by modulating intracytoplasmic Na+ in the cardiomyocyte. SGLT-2 inhibitors preserve mitochondrial function and reduce oxidative damage. They achieve this by inhibiting Na+/H+ exchanger 1 (NHE1) (SGLT-2 inhibitors inactivate NHE1 by binding to its Na+ binding site) and possibly SGLT-1 as well. All SGLT-2 inhibitors have intrinsic SGLT-1 blocking ability, albeit to different degrees.
Powering the Heart
Sodium-glucose cotransporter-2 inhibitors are able to switch myocardial metabolism away from anaerobic glycolysis toward utilization of ketones, free fatty acids (FFA), and branched-chain amino acids (BCAA). In a normally functioning heart, ketones, lactate, and BCAA are the situational sources of adenosine triphosphate (ATP) while glucose is the secondary source. The FFAs are the primary source.
In HF with T2DM, glucose becomes the primary fuel source, yielding lactate, which lowers the mitochondrial pH resulting in cardiomyocyte damage and dysfunction. Besides, there is reduced FFA metabolism resulting in steatosis and lipotoxicity. This results in reduced ATP and increased oxidative stress leading to cardiomyocyte damage and dysfunction.
Sodium-glucose cotransporter-2 inhibition increases the ketones which is an efficient source of ATP. Besides, glucose is displaced as a fuel source by ketones. Also, the FFA consumption is increased, thereby reducing steatosis and lipotoxicity, thus improving the myocardial energy supply and reducing toxicity.
Combination with Dipeptidyl Peptidase-4 Inhibitors in the Management of Diabetes
The rise in endogenous glucose production (EGP) with SGLT-2 inhibitors is directly proportional to the amount of glucosuria. It may happen that SGLT-2 inhibitors induce marked glucosuria when baseline glycated hemoglobin (HbA1c) is higher (≥8.5–9.0%), and this evokes a larger increase in EGP. This marked rise of EGP at higher baseline HbA1c may not be adequately suppressed by dipeptidyl peptidase-4 (DPP-4) inhibitors, and thus only a little benefit is observed when DPP-4 inhibitors are added. However, at moderate baseline HbA1c (<8.5%), SGLT-2 inhibitors-induced EGP was suppressible with DPP-4 inhibitors, and thus a combination of both these agents could produce a larger HbA1c reduction.
An increase in glucagon, decrease in insulin, and associated increase in EGP are clear disadvantages with SGLT-2 inhibitors. Interestingly, DPP-4 inhibitors decrease glucagon and increase insulin, and thus a combination of these agents appears rational and is expected to be synergistic.
Uric Acid-lowering Effect of SGLT-2 Inhibitors
It is mediated through GLUT9 and URAT-1.
Effects of SGLT-2 Inhibitors on Insulin Sensitivity and β-cell Function
The animal models have demonstrated improvement in fatty liver by alleviating hepatic inflammation and oxidative stress.
Anti-inflammatory Effects of SGLT-2 Inhibitors
Sodium-glucose cotransporter-2 inhibitors lower the levels of several cytokines such as tumor necrosis factor α (TNFα), interleukin-6, high-sensitivity C-reactive protein, and leptin.
SGLT-2 Inhibitors Reduce Renal Fibrosis and Enhance Erythropoietin Production
There is reduced partial oxygen pressure within the peritubular microenvironment. In animal models, SGLT-2 inhibitors administration restored oxygen partial pressure within the cortex, but the medullary oxygen partial pressure remained unchanged.
Modulation of Sympathetic Nervous System
Sodium-glucose cotransporter-2 inhibitors have also shown potential in modulating sympathetic activity, thereby breaking the vicious cycle of chronic sympathetic activation in patients with T2DM and HF. Large clinical trials, thus far, have shown no reflex increase in heart rate following blood pressure reduction from SGLT-2 inhibition, a surrogate marker for sympathetic blockade.
CONCLUSION
Sodium-glucose cotransporter-2 inhibitors have in recent times emerged as a powerful tool not only in the management of T2DM but also in in the management of HF, with or without diabetes, and diabetes with kidney disease. Besides, this class of drugs achieves these outcomes through varied mechanisms. They can be rationally combined with other classes of medications used in diabetes.
SUGGESTED READINGS
1. Wright EM, Hirayama BA, Loo DF. Active sugar transport in health and disease. J Intern Med. 2007;261(1):32-43.
2. Maack C, Lehrke M, Backs J, Heinzel FR, Hulot JS, Marx N, et al. Heart failure and diabetes: metabolic alterations and therapeutic interventions: a state-of-the-art review from the Translational Research Committee of the Heart Failure Association–European Society of Cardiology. Eur Heart J. 2018;39(48):4243-54.
3. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Eng J Med. 2019;380:347-57.
4. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Eng J Med. 2015;373(22):2217-8.
5. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcome in Type 2 diabetes mellitus and nephropathy. N Eng J Med. 2017;337:644-57.
6. Santos-Gallego CG, Requena-Ibanez JA, San Antonio R, Ishikawa K, Watanabe S, Picatoste B, et al. Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol. 2019;73(15):1931-44.
7. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31-9.
8. Toyama T, Neuen BL, Jun M, Ohkuma T, Neal B, Jardine MJ, et al. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: A systematic review and meta-analysis. Diabetes Obes Metab. 2019;21:1237-50.
9. Shi FH, Li H, Yue J, Jiang YH, Gu ZC, Ma J, Lin HW. Clinical adverse events of high-dose vs low-dose sodium-glucose cotransporter 2 inhibitors in type 2 diabetes: a meta-analysis of 51 randomized clinical trials. J Clin Endocrinol Metab. 2020;105(11):1-12.
10. Tanaka A, Node K. Clinical application of sodium-glucose cotransporter 2 inhibitor into a real-world setting of heart failure care. Cardiovasc Diabetol. 2020;19:132.
11. Damman K, Beusekamp JC, Boorsma EM, Swart HP, Smilde TDJ, Elvan A, et al. Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF). Eur J Heart Fail. 2020;22(4):713-22.
12. Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436-46.
13. Garg V, Verma S, Connelly K. Mechanistic insights regarding the role of SGLT2 inhibitors and GLP1 agonist drugs on cardiovascular disease in diabetes. Prog Cardiovasc Dis. 2019;62(4):349-57.
14. Fearnley CJ, Roderick HL, Bootman MD. Calcium signaling in cardiac myocytes. Cold Spring Harb Perspect Biol. 2011;3(11):a004242.
15. Min SH, Yoon JH, Moon SJ, Hahn S, Cho YM. Combination of sodium-glucose cotransporter 2 inhibitor and dipeptidyl peptidase-4 inhibitor in type 2 diabetes: a systematic review with meta-analysis. Sci Rep. 2018;8(1):4466.
 Home
Home
e-Contents
Chapter 1e: Prosthetic Valve Thrombosis
Harbir Kaur Rao, Rajinder Singh GuptaChapter 2e: Diabesity and Glucagon-like Peptide-1 Receptor Agonists
Rajiv Awasthi, Avivar AwasthiChapter 4e: Gestational Diabetes Mellitus: An Update
Vinay Kumar Meena, Nazim Hussain, Rajani NawalChapter 5e: Sodium-glucose Cotransporter-2 Inhibitors Beyond Glycemia
Amit VarmaChapter 6e: Intrahepatic Cholestasis
Archith Boloor, Nikhil Kenny ThomasChapter 8e: Tobacco and Chest
Rajbir Singh, Prabhpreet Kaur, BL Bhardwaj, RS BhatiaChapter 9e: Lung Metastasis
RS Bhatia, Prabhpreet Kaur, Rajbir Singh, BL BhardwajChapter 10e: Important Drug Interactions in Clinical Practice
Srirang AbkariChapter 11e: Renal Tubular Acidosis
Surjit TarafdarChapter 12e: Anemia in Chronic Kidney Disease
Saif Quaiser, Ruhi Khan, Shahzad Faizul HaqueChapter 14e: Current Positioning of Nonstatin Therapy of Dyslipidemia
Saumitra Ray, Srina RayChapter 15e: How to Deal with Complication of Prolonged Antibiotic Therapy
Pushpita MandalChapter 16e: Navigating End-of-life Medical Decisions with Cultural Sensitivity
Reinold OB GansChapter 17e: Osteocalcin: New Frontiers in Diabetes
Sudha Vidyasagar, Avinash HollaChapter 18e: Arterial Blood Gas Analysis: A Rational Approach
SV Ramanamurty, TVSP MurtyChapter 19e: Asthma: New Therapeutic Avenues
Sachin Hosakatti